亲,你被这个标题吓到了吗?
这是几个月前,美国FDA给大森林发来的一段留言。没吓到各位客户,倒是让大森林人吓到魂飞魄散。
2017年11月,大森林拼箱45HQ前往美国FTW1仓库,该柜中大部分货物需要做FDA REVIEW( FDA只是审核并不强制要求做FDA注册的货物),及另外一部分LED产品。
在货物到港之前,大森林美国报关行进行清关及将FDA REVIEW货物进行FDA申报。海关在接收到清关资料后24小时内给予放行,而由于有FDA监管货物,FDA部门暂时没有对他管辖内的货物放行,并向大森林报关行发送了一份电子HOLD货通知书:


此说明书说明的清清楚楚,
只有STAINLESS STELL FOOD SCISSORS
CUPPING THERAPY
MANUAL HANDHELD MASSANGER
BODY MASSANGER
货物需要等待FDA REVIEW
在收到此份通知书后,大森林海外仓迅速作出反应:将货物进行分拣,将FDA HOLD货物调出,其他放行货物先安排预约派送至FBA ,并等候FDA部门对上续四种产品进行审核,一般情况下,FDA会做文件审核后直接放行,另外一种为FDA会到货物存放现场验货后放行。
FDA官大人于1月6日来到大森林代理仓库进行验货。然而验货结束后,我们收到这样一份通知(一封来自大森林美国报关行的邮件):
LED’s were not at the facility and available for FDA exam. LED’s which are FDA regulated products must be transmitted with all future entries They advised that if we can request that the customer locate these goods and make them available for exam, if possible.
Also, they asked that we provide the actual manufacturer’s name and complete address for each FDA regulated items also if they have facility registration numbers also include them with our response.
So that we may know exactly what LED is, as it is too broad a term, please advise what this items is and please make sure invoices are detailed as when vague, it is causing too many problems
FDA查货现场居然声称为什么没有LED产品?
LED产品去哪里了?
I have detained 4 lines of this entry and will forward to our Center for Devices for review. However, there appears to be differing manufacturer information. The entry documents have one firm name, but the cartons have the name of Yongkang Jinkang Health Equipment Factory. I need to know as soon as possible the actual manufacturer of these devices. If the manufacturer is Yongkang, the Importer will need to provide an invoice from that manufacturer. If they are not, please provide an explanation as to why Yongkang is on the product labeling and outer cartons.
这封来自FDA的邮件,FDA声称产品的资料上提供的制造商和产品外箱制造商不符。 ( 这点大森林提议所有客户,特别是代理客户,一定如实申报美国制造商)
我们姑且忽略第二个FDA小问题,但第一个问题则让大森林人抓头皮了:在FDA扣货通知书上,哪个单词看到了LED呢? 拿望远镜也看不到LED啊。
但是官大神的话如同圣旨,在收到该邮件通知后,大森林火速通知客户下架LED产品,通通撤回到大森林代理仓库,进行最后的检验。想想这浩浩荡荡的退货,太雄伟了。(顿时也有种FDA官大人乱用私权的赶脚啊)
由于大批量退货导致客户产生巨额退货费,大森林面临来自客户沉重的压力。于是我们又开始了和FDA部门漫长的谈判过程。
沟通了无数个来回无果,FDA最后发来这么一段英文解释。
如下是来自FDA海关原始邮件配上大森林的翻译(顺带让大家体会纯正的美国英语):
LED lights are FDA regulated:(LED灯属FDA监管)
“LED products emit visible optical radiation which qualifies them to be a radiation emitting electronic product and unless it contains a laser, these LED products are not subject to a mandatory standard but the FDA does regulate them.
LED产品发射可见光辐射,使其成为辐射电子产品,除非它含有激光,这些LED产品不受强制性标准,但FDA对它们进行规范监管。
We recommend the manufacturer/importer to claim any product that we regulate and since we regulate all radiation emitting electronic products under the general/defect clause they should all be claimed. The LED manufacturers are subject to the ARO reporting requirement (21 CFR 1002.20) and notification if they discover a defect (21 CFR 1003). The product code we recommend the firms to transmit is shown below;
Since LEDs do not have a performance standard, a 2877 Form shouldn’t be required but you may still need to enter an Affirmation of Compliance of RA2 and state “There is no performance standard”.
我们要求制造商/进口商向我们申报任何属于我们规管的产品,因为根据一般/缺陷条款,我们规管的所有辐射的电子产品都应该申报。
LED厂商,如果发现一个缺陷(21 CFR 1003),受ARO报告要求(21 CFR 1002.20)和通知管制。我们建议公司使用的产品代码如下所示;
由于LED没有性能标准,可能不需要填写2788表格,但你可能依然需要填写一份表格,An Affirmation of Compliance of RA2 并声明“没有性能标准”。
ORA imports product code is:
ORA进口产品编码
非装置发光产品: LED, 照明,一般光学产品,非医疗用, 编码:95R--HH
Here is additional information on LED product code taken off our website:
下面是从我们网站上下载的关于LED产品的附加信息
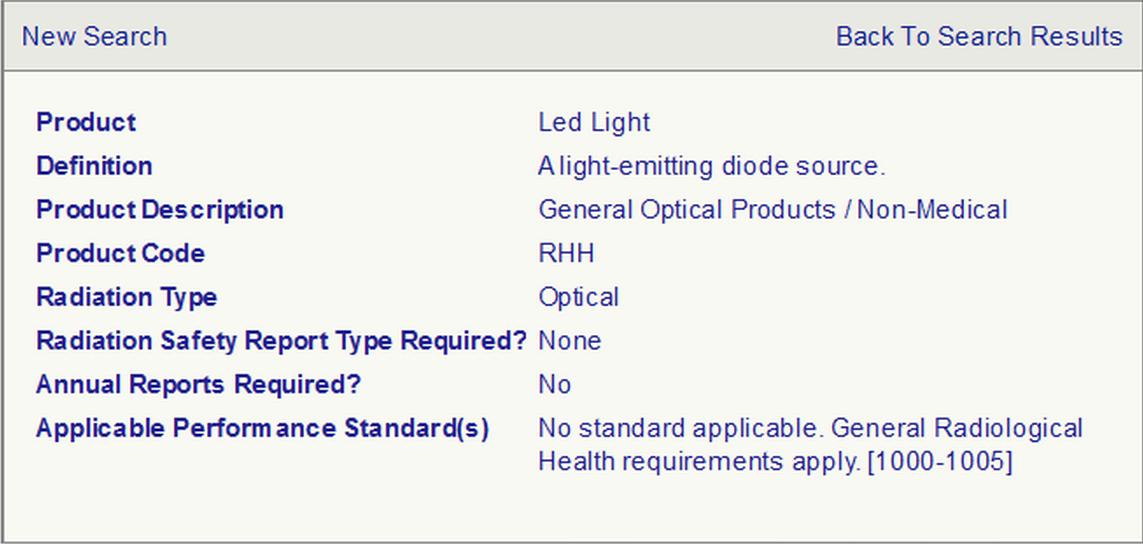
产品:LED 灯
定义:发光二极管光源
产品描述:一般光学产品/非医疗用
产品编码:RHH
辐射类型:光
是否需要辐射安全报告? 不需要
是否需要年度报告? 不需要
适用的性能标准:无适用标准。一般放射卫生要求(1000-1005)
Also, check out our website where it discusses LEDs: 可以查看FDA网站上关于论述LED产品的信息:
http://www.fda.gov/Radiation-EmittingProducts/ElectronicProductRadiationControlProgram/GettingaProducttoMarket/default.htm
Product Specific Questions
Q35) Are Light Emitting Diodes (LEDs) or Intense Pulsed Lights (IPLs) subject to the laser regulations and reporting?
LEDs and IPLs do not meet the definition of a laser, namely:
21 CFR 1040.10(b)(19) Laser means any device that can be made to produce or amplify electromagnetic radiation at wavelengths greater than 250 nm but less than or equal to 13,000 nm or, after August 20, 1986, at wavelengths equal to or greater than 180 nm but less than or equal to 1.0*106nm primarily by the process of controlled stimulated emission.
There is no existing FDA performance standard for LED or IPL products. They are not subject to Product or Annual reports under 21 CFR 1002. However, as they are radiation-emitting products, the manufacturers of these products would still be subject to the general requirements in Title 21 CFR 1000 through 1005, specifically, accidental radiation occurrence notifications and notifications of defect, 21 CFR 1003 & 1004.
产品具体问题
Q35)发光二极管(LEDs)或强脉冲光(IPLs)是否受激光法规和报告管制?
LEDs和IPLs不满足激光的定义,即:
21 CFR 1040.10(b)(19)激光设备,能够产生或放大电磁辐射的波长大于250 nm,但小于或等于13000纳米,1986年8月20日后,主要通过控制激光发射过程,在波长等于或大于180 nm,但小于或等于1.0* 106nm。
对于LED或者IPL产品,FDA没有现存的性能标准。根据21 CFR 1002,他们不受产品报告或年度报告管制。然而,因为它们是辐射发光产品,这些产品的制造商仍然受到21 CFR 1000的一般要求管制,具体来说,通过1005,做出意外辐射发生的通知和缺陷的通知,21 CFR 1003和。
如上美国FDA邮件以及大森林面临的这次退仓事件证明,最近传的沸沸扬扬的LED灯被FDA管制,总算有了终极答案: 只有用于医疗设备,带有激光辐射性的LED灯才会被FDA强制管制,而普通的LED灯并不是不被管制,而是FDA需要做REVIEW(审核),这意味着LED货物都需要进行FDA申报才能清关放行。也表示报关行要开始收FDA申报费了。
最后的最后,大森林强烈建议:为了减少不必要的麻烦和查验,LED产品在发票品名需注明 *For Dental Use* *For Industrial Use* *For Medical Use*(牙科用,工业用,医疗用。)这个非常重要,直接决定LED产品是只需要FDA申报还是需要FDA注册号。
当然,所有货物也务必真实提供制造商名称。
更多亚马逊报价及咨询,请关注大森林官方服务号:
